Engineering applications:
Isotopes having superfluous neutrons have a tendency to decay (to fire particles
and energy in the form of b or g radiation). The products of this splitting can be detected by
sensitive instruments (scintillation NaI or Si detectors have a resolution of 10-20 kg.m-3) and
this can be used, e.g., for the noninvasive monitoring of water flows in rivers, basins,
underground water, etc., see Thın (1998). This experimental technique makes use of tritiated
water as a tracer, which has exactly the same properties as the molecules of natural water and
therefore the "marked" molecules follow streamlines (trajectories going out from the inlet) for
example in a basin for biological treatment of waste water, Thın, itnı (1992), see Fig.
4.2.
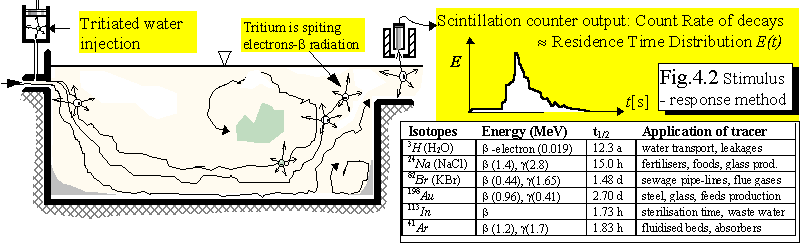
Detection of the tracer passage at the outlet tells us how long different particles flow through
the basin (this is called the residence time), see graph 4.2, describing the residence time
distribution. If the graph of the residence time distribution is very broad (spread, characterised
by a large value of standard deviation with respect to the mean residence time) we conclude
that the product of biochemical reaction will be very nonuniform and we should try to
improve the basin design (e.g., by changing the level of the inlet, inclining walls,
etc.)
Another interesting application of decaying isotopes is carbon dating , a
method often used by archaeologists. This method examines the presence of very slowly
decaying 14C isotope in objects that were living matter many thousands of years ago (the half-
life of 14C decay is 5730 years). From the relative mass of 14C in relation to stable
12C the age
of examined findings can be estimated. Time zero, the date of birth of the 14C isotope, is given
by the occurrence which comprises several chemical reactions, see, e.g., Holzclaw (1991):
isotope 14C originates in the upper atmosphere by transmutation from nitrogen as a result of
cosmic radiation (neutrons). 14C then reacts with oxygen to form CO2, which is absorbed by
plants and other living organisms (photosynthesis). As a result of these processes an
equilibrium between the content of 12C and 14C in organisms is established. When the plant
dies, it ceases to accept CO2 from the atmosphere, and the content of 14C starts to decrease.
@TEC: 3. 3.2003
Change language to


 peoples
peoples


 mailto: Zitny
mailto: Zitny
